People
Awarded student Hana Kopřivová aims to come up with a new system for diagnosing tumors
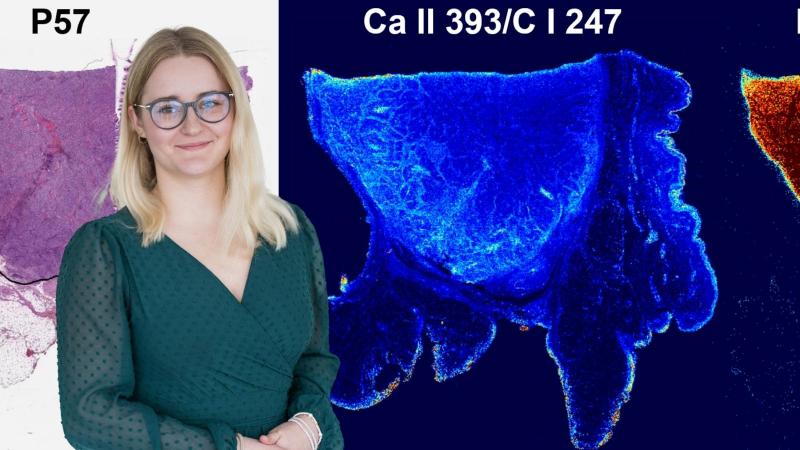
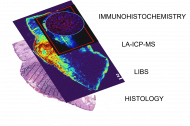
Her friends often ask her if they can show her their skin moles. She replies, amused, that she is not a skin doctor, and adds, in a warning tone, that they would not want their skin samples to ever end up in her lab. It wouldn't do any good. Hana Kopřivová from Professor Kaiser's research group (Advanced Instrumentation and Methods for Materials Characterisation) at CEITEC BUT works with skin samples from people whose health is being destroyed by cancer. In her project, she analyses different types of tumours using four techniques and tries to create an algorithm that would be able to recognise the tumour area based on the data obtained. The project is very multidisciplinary, using insights from chemistry, physics, computer science and medicine. The long-term goal is to reduce the risk in removing tumour tissue. The ambitious plan received the Brno Ph.D. Talent Award.
What does the award mean to you?
For me, the award represents much more than just a piece of paper. It is a confirmation that my work and efforts are worthwhile. It means that someone else sees the potential in what I do. This is not always the case in academia, which is why this award is a great recognition for me. It is also a motivation to keep working and developing my skills. The award lets me know that I am not doing something useless, but that my contribution can really make a difference and that others have noticed.
I read that two years ago you never imagined you would be a scientist. What's changed?
That's right. A lot of my college classmates said they would be attracted to a PhD, and I didn't understand. I didn't think I'd go on to academia. I liked the corporate style. Come in at 8:00 and go home at 16:00. And then I got a part-time job in the Laser Spectroscopy Lab, where I worked as a technician – measuring samples for other researchers. And I got hooked.
What did you study?
I studied Consumer Chemistry at the Faculty of Chemistry, BUT. And it was only during that part-time job at CEITEC, when they were applying for PhDs, that I was asked if I wanted to give it a try. That's when I realized that I enjoyed it, so I decided to continue and develop my work further.
In your PhD – studying biological tissues – you moved away from chemistry a bit.
When someone asks me what I'm actually studying now, it's not exactly easy to answer. In my group under Associate Professor Porizka, I'm doing spatial elemental mapping of biological soft tissues, so there's chemistry there. Plus we are half from the Faculty of Mechanical Engineering and half from CEITEC, so we have to learn about optics and physics, and a bit about data processing and computer science. Overall, it's very multidisciplinary. We're working on projects with biologists and doctors, so we're trying to get as much knowledge from different disciplines as possible. That way we can successfully collaborate with people from different fields. Although it's not exactly easy, we've been successful so far.
What is the goal of your project?
We have obtained a set of samples of various skin tumours, whether they are malignant tumours – melanomas, basal cell carcinomas, squamous cell carcinomas – or benign (non-malignant) tumours. Using four techniques (LIBS, LA-ICP-MS, immunohistochemistry, classical histopathology) we analyse them and try to correlate imaging. The outcome of the project would be an algorithm that would be "trained" from our samples and would be able to say – this is a tumor area, this is no longer a tumor area. But it's still very far in the future.
What would that mean?
Around the tumour tissue, a piece of the safety margin is always removed, actually the healthy skin. To make sure that everything can be removed. And if the tumour is still there, you have to have the operation again, but the tumour may have spread further in the meantime. In older people, frequent surgery may not be safe either. So we want to come up with a technique that lets doctors know that they've really cut out everything.
Where do you get human tissue from?
We got this kit from the University Hospital in Hradec Kralove. Imagine paraffin blocks with pieces of tissue cut out of them. We shoot lasers into them and analyse them. Tumour cells have a slightly different elemental composition than healthy tissue. With LIBS, we most often see magnesium in higher concentrations in tumours. It's still a hypothesis, but we are trying to show that magnesium could be a cancer biomarker. And because of that elemental composition, we're then able to tell where the tumor is and where it's not. But it's not that simple, sample by sample we have to consult pathologists because there are X types of tumors and each one behaves a little bit differently.
But it's not that excess magnesium is involved in cancer.
No, it certainly doesn't. What also plays a role is that cancer is described as uncontrolled cell division, so even in those tumors there is actually a higher density of cells and a higher concentration of elements than in healthy tissue. Another factor in the higher concentration of magnesium is other biological processes that I cannot explain in detail. That is up to the doctors. Of course, there may be more than one of these biomarkers, magnesium is just one example.
How many samples do you need to consult with pathologists to make machine learning as accurate as possible?
The more the better. Right now we're starting with a set that contains about 60–70 samples, which isn't enough. We're working on another collaboration that, if it works, will give us hundreds of samples, and that would be more promising, of course.
How do you work with the idea that you're working with a "piece" of a human being who is also sick?
Well... it's interesting. I'm not really aware of it. We only have pictures of the tumours, of course we don't know the identity of the patient. We don't know who it is. I don't think you have to admit it. It's not pleasant. We actually shoot lasers into the skin to create a micro-plasma from which we can collect the emitted radiation.
For whom would the results of your project make work easier? Doctors when examining moles?
If it ever succeeds in the future, it would serve pathologists. A dermatologist in a conventional surgery will not be able to do this at all, because we are talking about high-power lasers and it is not possible to shoot into a person's skin. We are always talking about tissue that has been removed from the human body.
I imagined it as some software that just "looks" at the mole and helps with the diagnosis.
It doesn't. It learns to evaluate tumors based on the information it learns from our samples. Classically, the tumor would be removed and sent to pathology first. The pathologist would have this pre-screening technique at hand that would tell him: here's the tumor and it's whole, but check it out. So it would be a two-stage check, which would reduce the chances of some kind of mistake that, for example, the patient would come back in two years' time with a piece of the tumour left in his body and he has to have surgery again and in the meantime it has metastasised elsewhere.
Does the research have an impact on the fact that you are constantly looking at your moles and monitoring their changes?
When I mention it somewhere, it's more like other people want to show me their moles (laughs).
What would you like to achieve in your focus?
That's a good question. I would be very happy if we could collect as many samples as possible so that we could validate the method and move as much as possible towards practical use. If there were, say, 300, 400, 500, which is extreme, then the results of the work would have enormous potential.
Is it difficult to obtain human tissue samples?
It's very difficult precisely because it's human tissue. So these are valuable samples. You have to justify everything to the ethics committee. Above all, you have to remember that you are holding a unique sample, there will never be another one like it. Which is sometimes stressful, because we burn human tissue in pieces with a laser, so we can only measure one sample twice at most and then it's gone. We have to be precise. Once we get it wrong, we lose all the data. We can't do it again.
Source: CEITEC BUT
High Entropy Oxides: Awarded PhD student Erik Ščasnovič works on research and development of new ceramic materials
Motor failures in electric vehicles can be detected by mathematical algorithms from the BUT
Simpler bone regeneration process. CEITEC researcher tests unique combination of titanium and hydroxyapatite
Women from BUT who move the world of science and technology
“I expected chaos, dirt and mess,“ says a researcher from CEITEC BUT who taught children in the Himalayas